
Starting date : October 2022
lifetime: 36 months
Program in support : European Innovation Council
Status project : in progress

Partners: EPFL, ONWARD, IICT-BAS (Institute of Information and Communication Technologies)
Target market: people currently living
with Spinal Cord Injury (SCI)
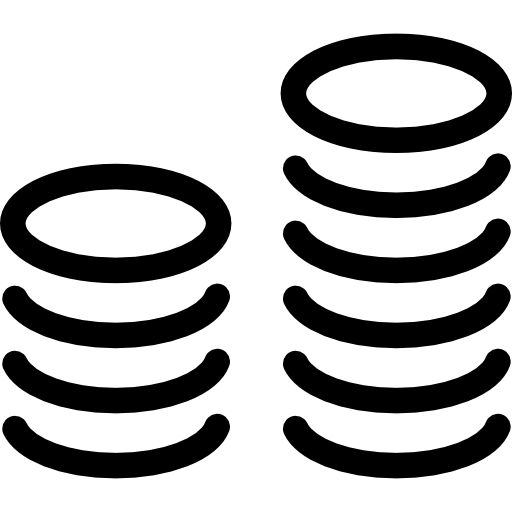
Investment: € 4.7 m.
Releases
[1] A. L. Benabid et al., “An exoskeleton controlled by an epidural wireless brain–machine interface in a tetraplegic patient: a proof-of-concept demonstration,” Lancet Neurol., Oct. 2019, doi: 10.1016/S1474-4422(19)30321-7.
[2] F. B. Wagner et al., “Targeted neurotechnology restores walking in humans with spinal cord injury,” Nature, vol. 563, no. 7729, Art. no. 7729, Nov. 2018, doi: 10.1038/s41586-018-0649-2.
[3] M. Capogrosso
et al., "A brain–spine interface alleviating gait deficits after spinal cord injury in primates,"
Nature, vol. 539, no. 7628, Art. no. 7628, Nov. 2016, doi: 10.1038/nature20118.
[4] A. B. Ajiboye et al., “Restoration of reaching and grasping movements through brain-controlled muscle stimulation in a person with tetraplegia: a proof-of-concept demonstration,” The Lancet, vol. 389, no. 10081, Art. no. 10081, May 2017, doi: 10.1016/S0140-6736(17)30601-3.
[5] B. Wodlinger, J. E. Downey, E. C. Tyler-Kabara, A. B. Schwartz, M. L. Boninger, and J. L. Collinger, “Ten-dimensional anthropomorphic arm control in a human brain-machine interface: difficulties, solutions, and limitations,” J. Neural Eng., vol. 12, no. 1, Art. no. 1, Dec. 2014, doi: 10.1088/1741-2560/12/1/016011.
[6] Lorach, H., Charvet, G., Bloch, J., & Courtine, G. (2022). Brain-spine interfaces to reverse paralysis. National Science Review.
Number of patents: n/a
| Stakes
-
PROBLEM.
746,000 people currently live with Spinal Cord Injury (SCI) in EU and in the US, with approximately 31,000 new cases in EU and 18,000 in the US each year, with dramatic human, societal and economical costs[1]. SCI leads to severe neurological deficits. Locomotion impairment or the loss of upper limb functions requires caregivers and dramatically impacts autonomy in every day life.
Currently, there is no approved therapy to improve motor recovery from SCIs.
[1]https://www.nscisc.uab.edu/public/2020%20Annual%20Report%20-%20Complete%20Public%20Version.pdf
BARRIERS.
Motor Brain Machine Interfaces (BMIs) aim at translating brain neural signals into commands to external
[1] or internal
[2] effectors using e.g. electrical stimulation of the spinal cord. In addition, as demonstrated in non-human primates, brain-controlled spinal cord stimulation may allow natural neuroprosthetic control and restore voluntary movements [3]. Short-term BMI proof-of-concepts
[4], [5] were milestones, but had the major limitation of using transcutaneous connectors. The ongoing clinical trials carried out by EPFL and CEA (STIMO-BSI - NCT04632290, and ‘BCI&Tetraplegia’ - NCT02550522) raise great hopes for SCI patients, effectively assessing the feasibility of chronic Electrocorticography (ECoG)-based motor BMI [6]. Wireless ECoG recording systems used in these clinical trials are far more appropriate for long-term application or during rehabilitation period. At the same time, these clinical trials highlight essential barriers to bring Motor BMI to clinical practice. Indeed, brain activity decoding to supply motor functions supposes a model in the decoding software, which requires a calibration.
The necessity of regular decoder retraining from neural activity recording, in a supervised manner and in well-controlled environments, is one of the major barriers for long-term use of BMI at home. In addition, due to their novelty, BMI systems are still an assembly of separate components including recording hardware (brain signals filtering / amplification / digitalization), data streaming and remote power supply hardware, dedicated computer with software to decode the digitalized brain signals and controlling the effectors. BMI systems are cumbersome, complicated to install, and often require a team of engineers to operate them
|
OBJECTIVES
This project aims at achieving 3 major methodological and technological breakthroughs toward assistance-free and easy to use portable neuroprosthetics with BMI signal processing integrated technology in medical practice (Fig. 1). The intended breakthroughs are: -
Auto-adaptive BMI framework: The usability of BMIs will be improved by alleviating the need of constant decoder recalibration. The first objective of
CEA LETI in the NEMO BMI project is the introduction of an auto-adaptive framework, which allows calibrating the neuronal activity decoder in adaptive manner in real time during the neuroprosthetics self-directed use. The innovative Auto-adaptive BMI (A-BMI) approach adds a supplementary loop with the decoder, which evaluates the level of coherence between user's intended motions and effector actions, based on neuronal data. It provides BMI task information (labels) to the data registered during the neuroprosthetics free use. Such data may be used for BMI system real-time training / calibration. Additionally, the A-BMI algorithms will be optimized following a neuromorphic approach with
IICT-BAS. The neuromorphic algorithms may improve BMI performance and be implemented in a novel chip with low-power consumption compared to conventional BMI algorithm hardware implementation.
-
Auto-adaptive brain-guided spinal cord electrical stimulation (by ONWARD and EPFL): Effectors using the patient's own limb through electrical stimulation of the spinal cord [2] offer a great benefit in terms of acceptability. However, the effector itself introduces some variability due to muscle fatigue, posture and level of neurological recovery. The variability originating from such effectors can impair the match between user's intentions and system output. The design of an auto-adaptive BMI framework will cope with the variability over the effector and will automatically optimize spinal cord stimulation. We will exploit the ongoing STIMO-BSI study (NCT04632290) where participants are implanted with a brain-controlled spinal cord system to demonstrate the benefits of such automated calibration process.
-
Miniaturization and portability based on integrated circuit technology: The design and fabrication of an integrated circuit for auto-adaptive, embedded, signal decoding is a milestone of the project. It would replace the computer used in current BMI systems. The objective for
CEA LIST here is to validate a technological brick (a miniaturized decoder). After validation during the course of this project, feasibility and specifications of a future fully-embedded neuroprosthetics will be assessed.
IMPACTS
- 746,000 people currently live with SCI in EU and in US, with approximately 31,000 new cases in EU and 18,000 in US each year . The majority of injuries (56%) occur between the ages of 16 and 30. While people with SCI confront a litany of health challenges and limitations to daily life activities, no efficient repair strategy to improve recovery after an SCI is yet available. “NEMO BMI intent is to decisively contribute to SCI patients motor impairment rehabilitation”.
- Thanks to the PATHFINDER CHALLENGE program, the development of new auto-adaptive algorithms will significantly contribute to enhancing knowledge on brain adaptation mechanisms. We foresee novelty in the design and implementation of neuromorphic hardware to sustain fast, secure, and low-power neuroprosthesis system functions beyond BMIs. Importantly, the consortium will follow an adaptive co-design and test strategy of hardware and algorithms (at a low TRL) that is based on the iterative use of online and offline clinical data. The NEMO BMI development model will become a gold standard for the successful development of adaptive neurotechnologies
|
|